Green River Cutthroat
Oncorhynchus virginalis ssp.
A fluvial Green River Cutthroat from a stream in Colorado.
Introduction
The Green River Cutthroat are a subspecies of Rocky Mountain Cutthroat Trout, native to the White, Yampa and upper Green River watersheds of Wyoming, Utah, and Colorado (Trotter et al. 2018). In addition, genetically unique populations of Green River Cutthroat are also found in several tributaries to the lower Colorado River, downstream of the confluence with the Green River including the Escalante and Fremont rivers in eastern Utah (Hepworth et al. 2001, Bestgen et al. 2019). Green River Cutthroat are often lumped in with Colorado River Cutthroat as a single subspecies (Behnke 1992, Behnke 2002, Trotter 2008), but represent one of three genetically distinct lineages found in the Colorado River drainage, termed the Blue (Green River Cutthroat), Green (Colorado River Cutthroat) and Red (San Juan Cutthroat) lineages (Metcalf et al. 2007, Metcalf et al. 2012, Rogers et al. 2018). The Green River Cutthroat and Colorado River Cutthroat appear to have diverged from each other approximately 1.2 to 1.7 million years ago and as such are genetically quite distinct from one another (Rogers et al. 2018, Shiozawa et al. 2018). Some authors indicate that the oldest type specimens for the Cutthroat of the Colorado and Green River watershed came from the Yampa River watershed in the Green River drainage and argue that the scientific name Oncorhynchus virginalis pleuriticus should be applied to the Green River Cutthroat (blue lineage) (Metcalf et al. 2012, Rogers et al. 2018). However, others indicate that specimens described by Cope (1872) to describe Salmo pleuriticus in fact came from Westslope Cutthroat in Idaho and Montana and suggest that the scientific name Oncorhynchus virginalis pleuriticus should be applied to the Colorado River Cutthroat (green lineage) to not add confusion to the recent proposed reclassification of Cutthroat Trout (Trotter et al. 2018). Today Green River Cutthroat are the most widespread of the three Cutthroat lineages in the Colorado River basin, in large part due to past stocking practices. Between 1903 and 1938, over 80 million pure Green River Cutthroat from Trappers Lake, in the upper White River of Colorado were widely stocked across Colorado and beyond and became established in many locations outside of their native range, including as far away as Williamson Lakes in California (Gold et al. 1978, Rogers et al. 2018). Due to this extensive stocking history nearly 20% of all Green River Cutthroat populations are found outside their native range and many of the Cutthroat populations in Colorado today can be linked to transplants from Trappers Lake (Rogers et al. 2018, Albeke 2020).
Life History Information
As with other subspecies of Rocky Mountain Cutthroat, the Green River Cutthroat express stream resident, fluvial and adfluvial life history strategies across their native range. While stream resident and adfluvial populations of Green Cutthroat are still found across their native range, fluvial or stream dwelling migratory populations have become relatively rare.
Stream Resident and Fluvial (River Migrant) Life History
Fluvial populations of Green River Cutthroat were once widespread throughout their native range but have been all but lost today and the stream resident life history is by far the most common and widespread life history form. Most stream resident populations remaining are found in small isolated high elevation streams with extremely short growing seasons. As a result, there is often little scope for growth in these populations and as such fish mature at small sizes. For example, the average Cutthroat in the headwaters of the North Fork Little Snake River, Wyoming matures at just 6” (15 cm) by age-3 and few reach sizes over 8” (20 cm) by age-6 (Quinlan 1980). Similar patterns have been observed in high elevation streams in the Uinta Mountains of Utah, where fish grow slowly and mature late. In these streams, the average age of maturity is 4 for males and 5 for females, which averaged just 7” (19 cm), additionally only 7% of the fish live past age 5, although Cutthroat as old as 12 have been observed (Belk et al. 2009). With such a limited scope for growth in most stream resident populations, Green River Cutthroat are highly opportunistic feeders, preying on whatever aquatic or terrestrial invertebrates are available. However, in more productive lower elevation streams, Green River Cutthroat may reach much larger sizes and fish in tributaries to the upper Green River in Wyoming with beaver ponds often reach sizes of 13” (34 cm)) or more and may also prey on the mottled sculpin that are also native to these streams (Young 1995). In high elevation streams, Green River Cutthroat prefer lower gradient reaches, with higher densities found in pools, and areas with undercut banks versus glides and riffles (Herger et al. 1996, Horan et al. 2000). Stream resident Green River Cutthroat in these small stream environments typically have small home ranges averaging just 105 ft (32 m) and spend over 70% of their time in pools, which they also rely on for overwintering habitat (Young 1998, Hodge et al. 2015).
Despite fluvial populations becoming rare across the range of the Green River Cutthroat, they do persist in some interconnected tributary streams. For example, 96% of the Cutthroat tracked during a study in a tributary to the Yampa River, Colorado exhibited a fluvial life history pattern (Hodge et al. 2017). Movement patterns of fluvial Cutthroat appear to vary depending on life stage specific habitat and growth needs. Juveniles, which prefer slower marginal habitat and have a higher scope for growth at warmer water temperatures predominantly move downstream, while older fish which have a lower scope for growth at higher temperatures, predominately move upstream in search of suitable habitat and water temperatures (Young 2011). Adult Cutthroat movements are also often seasonal and Cutthroat in the Yampa River tributary, migrated for spawning and to maximize growth opportunities by utilizing the lower elevation warmer stream reaches during the spring and fall and migrating 1.1 miles to 5 miles (1.8 km to 8 km) upstream to cooler water in the summer (Hodge et al. 2017). Fluvial life history patterns have also been observed in the Little Snake River, Wyoming, where Cutthroat migrate into tributaries in the spring to spawn, then drop back down to the river or utilize other tributaries during the summer (Trotter 2008). Over a period of two months, one fish in this watershed was observed descending a tributary where it was tagged, migrating downstream through the mainstem and into another tributary where it spent a week, before migrating back down to the mainstem and then moving upstream past the original tributary to hold throughout the summer (Young 1996). While fluvial fish in the Little Snake River move extensively during spring and fall, they become quite sedentary during the summer months and generally occupy home ranges of 1,000 feet (300 m) or less (Young et al. 1996). However, fish in the Yampa River tributary were much more mobile during the summer months with some moving up to 8.7 miles (14 km) (Hodge et al. 2017). During winter, Cutthroat in the upper Green River watershed in Wyoming, move into beaver ponds or deep scour pools where they remain for the season to avoid unstable stream conditions due to anchor ice (Lindstrom and Hubert 2004). While spawning plays a large role in the movements of Green River Cutthroat, maximizing growth is also important. Young et al. (1997) showed that fish from Little Snake River, fed primarily on terrestrial insects which are more energy rich than aquatic insects. By focusing on higher energy prey items and moving to maximize their scope for growth, fluvial Cutthroat are typically able to reach larger sizes than resident fish, with fish up to 18” (46 cm) found in some populations.
As with other Cutthroat trout, Green River Cutthroat are spring spawners and both stream resident and fluvial Green River Cutthroat typically spawn for the first time at age-2 for males and age-3 for females,although fish in some higher elevation populations may mature later. In most populations spawning occurs as runoff is subsiding, from mid-April to late-June depending on water temperature and elevation (Young 1996, Hodge et al. 2017). While Green River Cutthroat are able spawn multiple times, post-spawning mortality rates are typically high. Cutthroat that survive spawning, often remain in their upstream spawning habitat throughout the summer to avoid warm water temperatures downstream, only migrating back downstream as temperatures cool in the fall (Hodge et al. 2017). Juvenile Green River Cutthroat typically emerge from between mid-July and early-August and generally utilize slow and shallow stream margin habitat associated with large woody debris and pools in higher gradient streams during their first summer and fall (Bozek and Rahel 1991, Horan et al. 2000).
Adfluvial (Stream to Lake Migratory) Life History
Adfluvial populations once occurred in a number of small and relatively large lakes across the native range of the Green River Cutthroat, with major populations noted from Fish Lake, Utah, Fremont Lake, Wyoming and Trappers Lake, Colorado. Of these, the only large lake population remaining today, if in a slightly hybridized state is the Trappers Lake population, which is also the best studied adfluvial population. Trappers Lake is a 17,000-acre lake located at 9,633’ (2,936 m) at the headwaters of the White River in the Flattops Wilderness Area in Colorado and has been referred to as Colorado’s Yellowstone Lake due to its size, pristine environment, and famous Cutthroat fishery. The lake’s primary spawning tributary is Cabin Creek, and the trout ascend this stream as the ice recedes in early June, while spawners enter other tributaries one or two weeks later depending on the snowpack (Trotter 2008). Peak spawning generally occurs by late-June and continues until about the middle of July, with about 16% of the run consisting of repeat spawners (Snyder and Tanner 1960). Spawners in Trappers Lake typically range from age-2 to age-5, with about 80% of the run being age-3 and age-4 and the average female in the lake produces between 670 to 850 eggs at a size of about 12” (30 cm) (Trotter 2008). Cutthroat that survive spawning generally remain in the tributaries for about three weeks, before dropping back down into the lake in late July or August, although some remain in the tributaries until fall (Snyder and Tanner 1960, Trotter 2008). Fry begin emerging in late-July and begin migrating down to the lake to feed in mid-August to September, when they reach approximately 1” (2-3 cm), although a small proportion may remain in the stream over winter and migrate to the lake the following spring (Drummond and McKinney 1965). Once in the lake the Cutthroat are opportunistic and primary feed on scuds, and aquatic and terrestrial insects, with flying ants being an important food source in late summer (Trotter 2008). Cutthroat in Trappers Lake grow rapidly in their first year in the lake, typically reach approximately 9”, but growth slows significantly after that. As such the Trapper's Lake Cutthroat are not known for reaching very large sizes averaging about 14.5” (37 cm) at maturity but can reach a maximum size of about 18.5" (47 cm) with a maximum age of 8 (Babock 1969).
Not nearly as much is known about other native adfluvial Green River Cutthroat populations, but historic records indicate the Cutthroat in Fremont Lake, Wyoming, also were on the small side and were noted as being uniformly about 12” (30 cm), but extremely abundant. In contrast, Fish Lake in Utah, which is an extremely fertile lake with a significant prey base of snails, aquatic and terrestrial insects, leeches, scuds, daphnia, and mottled sculpin once produced Cutthroat in the 5 to 6 lb. (2.3 to 2.7 kg) class (Hazzard 1935, Trotter 2008). Similar to the trout in Trappers Lake, the Fish Lake Cutthroat were believed to begin spawning as soon as the ice receded in late May or early June, but little more is known about this population (Trotter 2008).
While there are relatively few remaining native adfluvial populations of Green River Cutthroat, they have been widely used for stocking alpine lakes and have established self-sustaining populations in many locations. Green River Cutthroat were stocked in Strawberry Reservoir in Utah for years and while they have since been replaced with Bear River Cutthroat there, we do know that the Green River Cutthroat in the reservoir once reached 12” to 14” long (30 to cm), much smaller than the current Bear River Cutthroat found in the reservoir. Green River Cutthroat in Strawberry Reservoir were noted for almost exclusively feeding on midges, with one trout having over 900 in its stomach (Hazzard and Madsen 1933), although they certainly also fed on other aquatic and terrestrial invertebrates. In the Escalante watershed in Utah, stream resident fish were transplanted into Dougherty Basin Lake and successfully established an adfluvial population. Fish in this lake spawn between late-May and mid-June, with females averaging 12” (31 cm) and producing and average of 582 eggs (Hepworth et al. 2000, Wagner and Oplinger 2013).
Status
Among the Cutthroat of the Colorado River basin, the Green River Cutthroat have fared the best, but has still suffered extensive declines over the past 150 years due to habitat degradation overharvest, and the introduction of non-native fish species. When Robert Behnke was searching for remnant Cutthroat populations in the basin in the 1960’s and 1970’s he indicated that he was only able to find two pure populations of Cutthroat in the Green River watershed, from two small streams in Wyoming (Behnke and Zarn 1976). Further searching in the area turned up 40 streams and two lakes that held Green River Cutthroat in Wyoming, but just 8 of these populations were considered genetically pure (Binns 1977). The disappearance of Green River Cutthroat was also echoed in northwestern Colorado, where Martinez (1988) reported that over the course of just 10 years, 12 of 37 populations became hybridized with Rainbow or other subspecies of Cutthroat Trout, 3 were replaced by Brook Trout and another was lost due to overharvest. As a result of their decline, calls were made for the Cutthroat of the Colorado River basin including the Green River Cutthroat to be listed under the Endangered Species Act (Behnke and Zarn 1976), which culminated in a petition to be listed in 1999 (USFWS 2000, USFWS 2004). Although this listing was determined to not be warranted (USFWS 2007), it prompted the states of Utah, Wyoming, and Colorado to develop a conservation strategy for Colorado River Cutthroat, which set milestones and prioritizes recovery actions for Cutthroat across the Colorado River basin (CRCT 2001). At the time that the conservation agreement was signed, Green River Cutthroat with less 10% hybrid influence were only believed to occur in 456 miles (734 km) of stream habitat and 14 lakes, accounting less than 5% of their native range, with Utah having the fewest populations of the three states (CRCT 2001). While stream surveys would turn up additional populations in the coming years, these fish had nearly vanished from their native range and the states set a goal to expand the distribution of Green River Cutthroat to 1,515 miles (2,439 km) of stream habitat and three additional lakes (CRCT 2001, Hirsch et al. 2005). This goal was further expanded in 2006 to reintroduce Green River Cutthroat to an additional 277.5 miles (446.5 km) of streams and two additional lakes (CRCT 2006). Today the Green River Cutthroat are in a much better spot than they were in the 1970’s, but to understand the Green River Cutthroat recovery efforts, it is important to first understand the factors that led to their decline in the first place.
The initial decline of the Green River Cutthroat can be linked to overharvest and habitat degradation. Green River Cutthroat require high quality habitat and as such as are negatively impacted by agricultural activities, mining, and logging. Much of the region where Green River Cutthroat are found is rich in ore and mineral deposits, and as such the region has an extensive history of mining, which left many streams contaminated with high levels of heavy metals and unable to sustain trout populations. These effects have persisted into the present day with high concentrations of lead and copper limiting Cutthroat abundance in the North Fork Little Snake drainage of Wyoming (Quinlan 1980, Jespersen 1981). However, in some cases the adverse effects of mining have served as a lifeline for the native Cutthroat by creating a toxic barrier that prevented nonnative species from invading (Young 1995). Logging has also impacted Green River Cutthroat due to a loss of riparian and upland vegetation and road building activities, resulting in increased water temperatures, sediment loads, more variability in stream flows and increased angler access to streams resulting in overfishing. Similar habitat effects can be seen from over-grazing, which often leads to a loss of riparian vegetation and unstable or incised stream banks, resulting in a loss of rearing habitat, warmer water temperatures and increased sediment loads which are often lethal to eggs incubating in the gravel. Other agricultural activities have also impacted Green River Cutthroat, with water withdrawals being a particular problem spot. Early irrigation withdrawals were typically unscreened, resulting in migratory Cutthroat entering canals and often failing to return to the streams before the canals were drained in the fall killing any remaining Cutthroat. Additionally, most water diversion structures were built without considerations for fish passage, thus blocking upstream and downstream movement in fluvial populations fragmenting the available habitat (Jespersen 1981, Young 1995). On top of that, the water rights associated with irrigation also often do not consider the need for instream flows, resulting in streams being overdrawn, reducing flows, and increasing water temperatures and thus limiting the abundance of native Cutthroat (Jespersen 1981). In additional to all the habitat related factors, Green River Cutthroat populations are particularly susceptible angling pressure and thus sensitive to overharvest. For example, one author reported catching 50 Cutthroat in 4 – 6 hours, while another survey indicated that anglers harvested at least a third of all the adult Cutthroat from a stream in Wyoming in a single year (Young 1995). These patterns of habitat degradation and overfishing have continued into recent times, and when fuel prices rose in the late 1970’s another resource extraction rush ensued to exploit the natural gas and oil reserves found across the native range of the Green River Cutthroat. In a predictable cycle, the early activities were poorly regulated, impairing Cutthroat habitat via road building while also resulting in an increase of angler access and effort along roads on these streams. On top of that the legal levels for releasing effluent contaminated with oil exceeded the lethal limits for Green River Cutthroat, further impairing populations (Trotter 2008). While Green River Cutthroat are resilient and able to withstand some level of habitat degradation and harvest, research indicates adult Cutthroat occur in significantly higher densities in streams in wilderness areas with a relatively small human footprint, compared to non-wilderness areas (Kershner et al. 1997).
As the effects of habitat degradation and overharvest, depleted populations of Green River Cutthroat, the response of early fisheries managers was to turn to hatchery supplementation and the introduction of nonnative species of salmonids. The most common species stocked within streams holding Green River Cutthroat, were Brook Trout, Brown Trout, Rainbow Trout and Yellowstone Cutthroat, while Lake Trout were often introduced to lakes hosting adfluvial populations. Green River Cutthroat evolved in isolation of other salmonids, and such do not deal well with any of these invaders. Additionally, each of these species exerts a unique negative effect that the native Cutthroat are poorly suited to deal with. Lake Trout and Brown Trout are both capable of reaching large sizes and are highly piscivorous, readily preying on the smaller native Cutthroat and either significantly reducing or eliminating the Cutthroat population they are interacting with. Both Brook Trout and Brown Trout spawn in the fall, which means that their offspring emerge in the spring giving them a clear competitive advantage over the spring spawning Cutthroat, whose offspring don’t emerge until late summer. This competition does not end at the juvenile life stage either, with both species relying on similar habitats. De Staso and Rahel (1994) showed that while adult Green River Cutthroat and Brook Trout were nearly equal competitors at 50 °F (10 °C), when the water temperature warmed to 68 °F (20 °C), Brook Trout showed clear competitive dominance, becoming more aggressive, consuming more food, and occupying the lead position in a dominance hierarchy more often than the Cutthroat. In one case a single introduction of Brook Trout into a high mountain lake in the 1950's eliminated the entire population of Green River Cutthroat in Battle Creek, Wyoming (Young 1995). Rainbow Trout and other subspecies of Cutthroat (primarily Yellowstone Cutthroat) also negatively affect with Green River Cutthroat via competition and predation, but more significantly both are capable of spawning with Green River Cutthroat and producing viable offspring, resulting in the replacement of native populations completely after repeated stocking or a loss of genetic integrity of native populations due to hybridization. In high elevation streams in Utah, Rainbow Trout and Yellowstone Cutthroat were often stocked at 10” to 12” (25 to 30 cm), which is larger than the maximum size of most Green River Cutthroat. As a result, this increases the likelihood of these nonnative trout to take the best feeding areas and offers males a competitive edge over the native Cutthroat during spawning, increasing the chance of hybridization (Belk et al. 2009). While stocking nonnative species in waters holding native Cutthroat has been all but completely curtailed today, many of these nonnative species are now firmly established on the landscape and continue to invade waters holding native Cutthroat.
One of the most common solutions to preserve Green River Cutthroat populations from invading nonnative salmonids has been to construct barriers to isolate Cutthroat populations from the nonnative trout. While this can be effective at isolating the Cutthroat, it also comes with its own suite of consequences. First, if there is not enough habitat upstream of the barrier, it prevents Cutthroat from expressing migratory life histories, disrupting access between overwintering, spawning and foraging habitats that allow Cutthroat to reach larger sizes and thus have a higher reproductive output (Novinger and Rahel 2003). Second, isolation in a small amount of habitat also results in small effective population sizes for Cutthroat populations, which can make them vulnerable to loss of genetic diversity and increase their eventual risk of extirpation (Cook et al. 2010). These barriers and nonnative trout removal projects are also costly and do not always result in the desired outcomes. For one, the nonnative species are often popular with anglers and without proper public outreach there is a real risk of nonnative species being reintroduced to the habitat upstream illegally. In other cases, such as Labarge Creek in Wyoming, the reintroduced Cutthroat population upstream of the barrier may fail to reestablish a viable natural population.
In 1999, Labarge Creek was selected by the Wyoming Game and Fish Department (WGFD) as a priority location to reintroduce native Green River Cutthroat due to its large amount of high-quality interconnected habitat. By 2007 a barrier had been constructed and all nonnative trout had been removed from 58 miles (93 km) of the watershed and WGFD started planting up to 50,000 Cutthroat in the creek annually to support the reintroduction. However, despite this effort, the hatchery plants have experienced exceptionally high mortality rates, natural reproduction by Cutthroat in the creek has been minimal and trout densities remain much lower than those displayed by the nonnative trout prior to the start of the project. It is not completely clear what factors have contributed to the lack of success at Labarge Creek, although the primary drivers are believed to be either domestication effects due to hatchery rearing or the use of an adfluvial stock from North Piney Lakes, when Labarge Creek is best suited to a fluvial population (LeCheminant et al. 2021) and it can only be hoped the future changes in the recovery efforts in the watershed will prove more effective.
While the small size and remote, isolated nature of many stream resident populations allowed them to persist into the present day, the adfluvial or fluvial life histories have been all but eliminated today. Fish Lake, Utah which was once famed for its productive Cutthroat fishery provides an example of how fast a highly exploited Cutthroat population could disappear. Beyond the issues already discussed, as hatcheries rose to prominence in the early 1900’s many adfluvial Cutthroat populations, were utilized extensively as egg-taking stations, with little thought for maintaining the population in their native waters. This was particularly true in Fish Lake, where on top of an extremely high-level of fishing pressure, 4,000,000 eggs were taken between 1901 and 1902 with extensive egg take operations also occurring in the proceeding years (Singler and Singler 1986). Despite being exceptionally productive, the combination of excessive egg-takes and overfishing, soon resulted in the Cutthroat population becoming depleted and as a result, a number of non-native species such as Brook Trout, Lake Trout, Rainbow Trout and Chinook and Coho Salmon were introduced to the lake. While the Chinook and Coho failed to become established, Brook, Lake and Rainbow Trout did, and between competition, predation, and hybridization by 1933 only 20 Cutthroat entered the fish traps on the lake and within a few short years they had vanished entirely (Hazzard 1935, Trotter 2008). While Fremont Lake in Wyoming escaped the egg-taking pressure, it followed a similar fate to Fish Lake after non-native species such as Lake Trout were introduced and the native Cutthroat silently faded into extinction.
Of the adfluvial populations, Trappers Lake remains the last great stronghold for adfluvial Green River Cutthroat, but it is not immune to the nonnative trout invasions and other issues that have wiped out so many other populations. Similar to Fish Lake, Trappers Lake severed as the primary egg-take station for Cutthroat across Colorado beginning in 1914, and by the 1920’s over two million eggs were being taken annually, while maintaining a robust Cutthroat population in the lake (Rogers and Wangnild 2005). However, in 1937 the Cutthroat population in the lake crashed resulting in egg-takes being halted. To rebuild the population and supplement the fishery, Yellowstone Cutthroat were planted in the lake. Between 1943 and 1950, close to a million Yellowstone Cutthroat were introduced into the lake, resulting in a hybridization 50% of the native Cutthroat population (Roger and Wangnild 2005, Trotter 2008). Another looming threat to Trappers Lake Cutthroat were Brook Trout, which had been stocked into Crescent Lake located just a half mile from Trappers Lake. While Crescent Lake typically does not have an outlet, in 1984 a high-water year resulted in the lake overflowing and allowed Brook Trout to reach Trappers Lake. Within two decades, Brook Trout already accounted for 40% of the trout in Trappers Lake, resulting in CPW biologist instituting a costly trapping effort to suppress the Brook Trout population and keep it from expanding further. On top of the impacts from Brook Trout, whirling disease was detected the lake in 2003. Whirling disease, which was introduced to North America from Europe in the 1950’s was first discovered in Colorado in the early 1990’s and has now spread throughout much of the state. The disease is caused by a parasite that requires two hosts for its lifecycle, tubifex worm which feeds on decomposing organic materials (such as dead trout) and trout. Worms infected with whirling disease exude spiked spores that attach to juvenile fish and inject cells into the fish that burrow through their flesh seeking cartilage in the skull and spine. This results in a variety of symptoms in fish including blackening of the skin, a shriveled tail, deformed skull or creating pressure on the spine and brain stem causing fish to swim in circles (i.e. whirling) often killing the trout outright. This disease is particularly problematic as it also primarily effects the juvenile life stages that are already under immense stress in Trappers Lake due to competition with Brook Trout. It is unclear how the disease reached the lake, but as it was already found in the White River downstream, it was most likely a hitchhiker on an angler’s waders or gear. Regardless of how it arrived, the disease is another pressure impacting, what was once one of the crown jewel Green River Cutthroat populations. However, while Cutthroat in Trappers Lake are struggling, the genetics of the population have been preserved due to the extensive transplants that occurred over the years. Today genetically pure populations of Trappers Lake Cutthroat persist in Williamson Lakes, California from a transplant in 1931 and Nanita Lake in Rocky Mountain National Park from a transplant in 1933, prior to the introduction of Yellowstone Cutthroat (Gold et al. 1978, Rogers and Wangnild 2005). Nanita Lake has subsequently been used to establish a hatchery broodstock of pure Trappers Lake strain Cutthroat used for stocking alpine lakes across Colorado.
To address the drastic decline in the Cutthroat population and fishery at Trappers Lake, CPW established a recovery plan for the lake in 2005 (Rogers and Wangnild 2005). This plan is multifaceted, with the first component to re-establish pure Trappers Lake stock fish from Nanita Lake into Trappers Lake. To do this, the plan calls for the state to install a fish trap on the primary spawning stream, Cabin Creek to prevent hybridized Cutthroat from spawning, and use barriers to trap out-migrating juveniles to be screened for hybridization (Rogers and Wangnild 2005). On top of the removal efforts, plan also tasks the state with stocking the lake with pure strain Trappers Lake Cutthroat from Nanita Lake to supplement the lake and boost the genetic purity of the population. Additionally, the plan includes components to protect pure populations in headwater lakes and tributaries upstream of the lake and reclaim any populations hosting nonnative trout or hybrids. The second component of the plan involves to controlling the Brook Trout population in the Trappers Lake. using a combination of fishing regulations that encourage anglers to keep Brook Trout, as well as directed removal efforts using Fyke nets targeting spawning fish in the fall and potentially the use of electrofishing to remove juveniles if necessary (Rogers and Wangnild 2005). The wild card in the recovery effort is whirling disease, which is not believed to have led to a population level decline yet but is being closely monitored by the state. It is unclear how successful these efforts have been to date, but Trappers Lake certainly have a long road ahead if it hopes to regain its glory as Colorado’s premiere native Cutthroat fishery.
While Labarge Creek and Trappers Lake provide examples of the ongoing challenges of conserving Green River Cutthroat populations, there are success stories and progress as well. One such place is in North Creek, which is one of the primary tributaries to the Escalante River in Utah. Extensive searches in the basin resulted in the discovery of 7 remaining native populations between the 1980’s and 2011, including a small pure population in a tributary to North Creek in the late 1990’s (Hepworth et al. 2001, Hadley 2014). In the mid-2000’s biologists successfully removed the nonnative trout from the upper reaches of North Creek and reintroduced fish from the pure population to the basin. Then in 2019, Utah set its sights on a more ambitious plan to restore Cutthroat to an additional 10 miles (15.7 km) of habitat in the basin including two reservoirs. This effort is currently still in progress, but in 2022 biologists successfully removed nonnative trout from 7.9 miles (12.7 km) of the stream and 23 acres of lakes, with additional removals and the reintroduction of Cutthroat to the stream set for 2023 (Hadley and Golden 2023). While Green River Cutthroat only inhabited 456 miles (734 km) of stream habitat in 2001, as of the last status review in 2015, they occupied 1,564 miles (2,517 km) of stream habitat or 16.6% of their native range (Albeke 2020). In particular, Utah which hosted the fewest conservation populations when the conservation strategy was put in place with just 177 miles (285 km) of stream habitat occupied has expanded Green River Cutthroat occupation to 854 miles (1,374 km) of stream habitat (CRCT 2001, UDWR 2020). There are still challenges to be faced such as the work at Trappers Lake and Labarge Creek, but it is also clear that a significant amount progress has been made in just 20 years and with additional recovery efforts in the works, the future of this subspecies looks much brighter going into the future.
Description
The Green River Cutthroat and Colorado River Cutthroat are similar in appearance, and due to the extensive stocking of Green River Cutthroat into the native range of the Colorado River Cutthroat morphological differences between the two lineages went unrecognized until very recently. It has only been with the application of genetics to determine the lineage of the populations of Cutthroat across the southern Rocky Mountains that the distinctiveness of these two subspecies has emerged (Metcalf et al. 2012, Bestgen et al. 2019). Compared to Colorado River Cutthroat, the Green River Cutthroat have more, and smaller spots spread across their body, with populations in the upper Green River watershed noted for particularly small spots while populations from the Little Snake River in the Yampa drainage have much larger spots. While these spots are often concentrated above the lateral line and towards the posterior part of the body, they may be distributed quite evenly across the body on some individuals. The coloration of the Green River Cutthroat varies from population to population, but they typically have greenish-brassy colored backs, which transition to an orangish-yellow or bronze-gold color along the sides. Some individuals exhibit a band of pink, orange or red along their lateral line and an orangish-red color on their gill plates. Additionally, the bellies on some fish, especially sexually mature males may be a bright orange or a vibrant red color. Juveniles exhibit 8-11 oval shaped parr marks on their sizes, which fade as they reach maturity. Like all Cutthroat, the Green River Cutthroat exhibits cutthroat slash marks under the jaw that are typically a vibrant orange or red color.
Taxonomic Characteristics: Gill rakers average 19.7, with a range of 18-20. Pyloric Caeca average 37, with a range of 34-43. Scales along lateral line average 198, with a range of 185-217. Basibranchial teeth average 8, with a range of 3 to 24 (Bestgen et al. 2019).
Stream Resident Form - Upper Green River Drainage
Click on images to view a larger picture
Fluvial Form - Yampa River Drainage
Click on images to view a larger picture
Rainbow Trout x Green River Cutthroat Hybrids
Click on images to view a larger picture
Former Greenback Cutthroat - Colorado River x Green River Hybrids
Click on images to view a larger picture
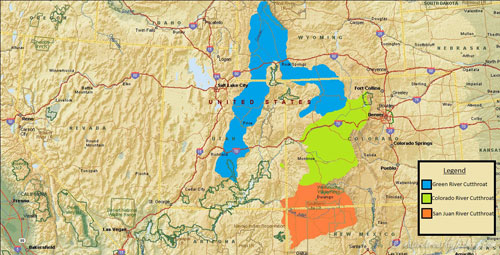
Native Range
A map of the original native range of the Green River (Blue Lineage), Colorado River Cutthroat (Green Lineage) and San Juan Cutthroat Trout. Data Source: Behnke (2002) and Trotter (2008). Data Source: Behnke (2002) and Trotter (2008).Below: A map of the native range of the Green River Cutthroat Trout.
References
Albeke, S.E. 2020. Addendum: updated range-wide status information for Colorado River Cutthroat Trout for the period 2011-2015. Colorado Division of Wildlife, Fort Collins.
Babcock, W.H. 1969. Growth and annuli of jaw-tagged Cutthroat Trout. Progressive Fish-Culturist 31(4): 215-216. https://doi.org/10.1577/1548-8640(1969)31[216:GAAOJC]2.0.CO;2
Behnke, R. J. 1992. Native trout of western North America. American Fisheries Society Monograph 6. American Fisheries Society, Bethesda, Maryland.
Behnke, R. 2002. Trout and Salmon of North America. Chanticleer Press, New York.
Behnke, R.J. and M. Zarn. 1976. Biology and management of threatened and endangered western trouts. USDA Forest Service, Rocky Mountain Forest and Range Experiment Station, Fort Collins, Colorado. General Technical Report RM-28.
Belk, M.C., M.N. McGee, D.K. Shiozawa. 2009. Effects of elevation and genetic introgression on growth of Colorado River Cutthroat Trout. Western North American Naturalist 69(1): 56-62. https://doi.org/10.3398/064.069.0116
Bestgen, K.R., K.B. Rogers and R. Granger. 2019. Distinct phenotypes of native cutthroat trout emerge under a molecular model of lineage distributions. Transactions of the American Fisheries Society 148:442-463. https://doi.org/10.1002/tafs.10145
Binns, N.A. 1977. Present status of indigenous populations of cutthroat trout, {Salmo clarki), in south-west Wyoming. Wyoming Game and Fish Department, Cheyenne. Fisheries Technical Bulletin 2.
Bozek, M.A. and F.J. Rahel. 1991a. Assessing habitat requirements of young Colorado River cutthroat trout by use of macrohabitat and microhabitat analyses. Transactions of the American Fisheries Society 20:571-581. https://doi.org/10.1577/1548-8659(1991)120<0571:AHROYC>2.3.CO;2
Cook, N. F.J. Rahel and W.A. Hubert. 2010. Persistence of Colorado River Cutthroat Trout populations in isolated headwater streams of Wyoming. Transactions of the American Fisheries Society 139: 1500–1510 https://doi.org/10.1577/T09-133.1
Cope, E.D. 1872. Report on the recent reptiles and fishes of the survey, collected by Campbell Carrington and C.M. Dawes. Pages 467-476 in F.V. Hayden, editor. Fifth annual preliminary report U.S. Geological Survey of Montana and portions of adjacent territories. Government Printing Office, Washington, D.C.
CRCT Task Force. 2001. Conservation agreement and strategy for Colorado River cutthroat trout (Oncorhynchus clarki pleuriticus) in the states of Colorado, Utah, and Wyoming. Colorado Division of Wildlife, Fort Collins, Colorado.
CRCT Coordination Team. 2006. Conservation strategy for Colorado River cutthroat trout (Oncorhynchus clarkii pleuriticus) in the States of Colorado, Utah, and Wyoming. Colorado Division of Wildlife, Fort Collins. 24p.
Drummond, R.A. and T.D. McKinney. 1965. Predicting the recruitment of cutthroat trout fry in Trappers Lake, Colorado. Transactions of the American Fisheries Society 94(4): 389-393. https://doi.org/10.1577/1548-8659(1965)94[389:PTROCT]2.0.CO;2
Gold, J.R., G.A.E. Gall and S.J. Nicola. 1978. Taxonomy of the Colorado Cutthroat Trout (Salmo clarki pleuriticus) of the Williamson Lakes, California. California Fish and Game 64(2): 98-103.
Hadley, M.J., M.E. Golden and J.E. Whelan. 2014. 2013 survey of Colorado River Cutthroat Trout in the Escalante and Fremont River drainages, Utah. Publication Number 14-08. Utah Division of Wildlife Resources. Salt Lake City, Utah.
Hadley, M.J. and M.E. Golden. 2023. Colorado River Cutthroat Trout resotration in the North Creek drainage: 2022 activities. Utah Division of Wildlife Resources. Salt Lake City, Utah.
Hazzard, A.S. and M.J. Madsen. 1933. Studies of the food of the cutthroat trout. Transactions of the American Fisheries Society 63:198–203.
Hazzard, A.S. 1935. A preliminary study of an exceptionally productive trout water, Fish Lake, Utah. Transactions of the American Fisheries Society 65(1): 122-128. https://doi.org/10.1577/1548-8659(1935)65[122:APSOAE]2.0.CO;2
Hepworth, D.K., M.J. Ottenbacher and C.B. Chamberlain. 2000. Trapping and spawning Colorado River Cutthroat Trout at Dougherty Basin Lake, 1999, an initial effort. Publication Number 00-16. Utah Division of Wildlife Resources. Salt Lake City, Utah.
Hepworth, D.K., M.J. Ottenbacher and C.B. Chamberlain. 2001. Occurrence of native Colorado River cutthroat trout (Oncorhynchus clarki pleuriticus) in the Escalante River drainage, Utah. Western North American Naturalist 61(2): 129–138.
Herger, L.G., W.A. Hubert and M.K. Young. 1996. Comparison of habitat composition and Cutthroat Trout abundance at two flows in small mountain streams. North American Journal of Fisheries Management 16: 294-301. https://doi.org/10.1577/1548-8675(1996)016<0294:COHCAC>2.3.CO;2
Hirsch, C. L., T. P. Nesler, and S. Q. Albeke. 2005. Range-wide status of Colorado River cutthroat trout. (Oncorhynchus clarkii pleuriticus) Colorado River cutthroat trout Conservation Coordination Team Report. Craig, Colorado.
Hodge, B.W., R. Henderson, K.B. Rogers and K.D. Battige. 2015. Efficacy of portable PIT detectors for tracking long-term movement of Colorado River Cutthroat Trout in a small montane stream. North American Journal of Fisheries Management 35(3): 605-610. https://doi.org/10.1080/02755947.2015.1012280
Hodge, B.W., K.D. Battige and K.B. Rogers. 2017. Seasonal and temperature-related movement of Colorado River Cutthroat Trout in a low-elevation, Rocky Mountain stream. Ecology and Evolution 7(7): 2346-2356. https://doi.org/10.1002/ece3.2847
Horan, D.L., J.L. Kershner, C.P. Hawkins and T.A. Crowl. 2000. Effects of habitat area and complexity on Colorado River cutthroat trout density in Uinta Mountain streams. Transactions of the American Fisheries Society 129:1250–1263. https://doi.org/10.1577/1548-8659(2000)129<1250:EOHAAC>2.0.CO;2
Jespersen, D.M. 1981. A study of the effects of water diversion on the Colorado River Cutthroat Trout (Salmo clarki pleuriticus) in the drainage of the North Fork of the Little Snake River in Wyoming. Masters Thesis. University of Wyoming. Laramie, Wyoming.
Kershner, J.L., C.M. Bischoff and D.L. Horan. 1997. Population, habitat, and genetic characteristics of Colorado River cutthroat trout in wilderness and nonwilderness stream sections in the Uinta Mountains of Utah and Wyoming. North American Journal of Fisheries Management 17:1134-1143. https://doi.org/10.1577/1548-8675(1997)017<1134:PHAGCO>2.3.CO;2
LeCheminant, A.G. G.M. Barrile, S.E. Albeke and A.W. Walters. 2021. Movement dynamics and survival of stocked Colorado River Cutthroat Trout. Transactions of the American Fisheries Society 150(6): 679-693. https://doi.org/10.1002/tafs.10322
Lindstrom, J.W. and W.A. Hubert. 2004. Ice processes affect habitat use and movements of adult Cutthroat Trout and Brook Trout in a Wyoming foothills stream. North American Journal of Fisheries Management 24: 1341-1352. https://doi.org/10.1577/M03-223.1
Martinez, A.M. 1988. Identification and status of Colorado River cutthroat trout in Colorado. American Fisheries Society Symposium 4:81-89.
Metcalf, J.L., V.L. Pritchard, S.M. Silvestri, J.B. Jenkins, J.S. Wood, D.E. Cowley, R.P. Evans, D.K. Shiozawa and A.P. Martin. 2007. Across the great divide: genetic forensics reveals misidentification of endangered cutthroat trout populations. Molecular Ecology 16(21): 4445-4454. https://doi.org/10.1111/j.1365-294X.2007.03472.x
Metcalf, J.L., S.L. Stowell, C.M. Kennedy, K.B. Rogers, D. McDonald, J. Epp, K. Keepers, A. Cooper, J.J. Austin and A.P. Martin. 2012. Historical stocking data and 19th century DNA reveal human induced changes to native diversity and distribution of cutthroat trout. Molecular Ecology 21(21): 5194-5207. https://doi.org/10.1111/mec.12028
Novinger, D.C. and F.J. Rahel. 2003. Isolation management with artificial barriers as a conservation strategy for cutthroat trout in headwater streams. Conservation Biology 17(3): 772-781. https://www.jstor.org/stable/3095235
Quinlan, R.E. 1980. A study of the biology of the Colorado River cutthroat trout (Salmo clarki pleuriticus) population in the North Fork of the Little Snake River drainage in Wyoming. Master's thesis. University of Wyoming, Laramie.
Rogers, K.B., K.R. Bestgen, S.M. Love Stowell and A.P. Martin. 2018. Cutthroat trout diversity in the southern Rocky Mountains. Pages 323-341 in P. Trotter, P. Bisson, L. Schultz and B. Roper, editor. Cutthroat Trout: evolutionary biology and taxonomy. American Fisheries Society, Special Publication 36, Bethesda, Maryland.
Shiozawa, D. K. , R. P. Evans, D. D. Houston, and P. J. Unmack. 2018. Geographic variation, isolation, and evolution of Cutthroat Trout with comments on future directions for management and research. Pages 129-172 in P. Trotter, P. Bisson, L. Schultz, and B. Roper, editors. Cutthroat Trout: evolutionary biology and taxonomy. American Fisheries Society, Special Publication 36, Bethesda, Maryland.
Singler, J.W. and W.F. Singler. 1986. History of fish hatchery development in the great basin states of Utah and Nevada. The Great Basin Naturalist 46(4): 583-594.
Snyder, G.R. and H.A. Tanner. 1960. Cutthroat trout reproduction in the inlets to Trappers Lake. Technical Bulletin 7. Colorado Department of Game, Fish and Parks. Denver, Colorado.
Trotter, P. 2008. Cutthroat: Native Trout of the West. Second Edition. University of California Press, Berkley, CA.
Trotter, P., P. Bisson, B. Roper, L. Schultz, C. Ferraris, G.R. Smith and R.F. Stearley. 2018. A special workshop on the taxonomy and evolutionary biology of cutthroat trout. Pages 1-31 in Trotter P, Bisson P, Schultz L, Roper B (editors). Cutthroat Trout: Evolutionary Biology and Taxonomy. Special Publication 36, American Fisheries Society, Bethesda, Maryland.
Underwood, Z.E., C.A. Myrick and K.B. Rogers. 2012. Effect of acclimation temperature on the upper thermal tolerance of Colorado River cutthroat trout Oncorhynchus clarkii pleuriticus: thermal limits of a North American salmonid. Journal of Fish Biology 80: 2420-2433. https://doi.org/10.1111/j.1095-8649.2012.03287.x
UDWR. 2020. Utah Division of Wildlife Resources Colorado River Cutthroat Trout conservation strategy. Publication Number 20-19. Utah Division of Wildlife Resources. Salt Lake City, Utah.
USFWS (U.S. Fish and Wildlife Service). 2000. A petition for rules to list the Colorado River cutthroat trout (Oncorhynchus clarki pleuriticus) as threatened or endangered under the Endangered Species Act. Center for Biological Diversity, Tucson, Arizona.
USFWS (U.S. Fish and Wildlife Service). 2004. 90-Day finding on a petition to list the Colorado River Cutthroat Trout. Federal Register 69:21151.
USFWS (U.S. Fish and Wildlife Service). 2007. 12-Month finding for a petition to list the Colorado River Cutthroat Trout as Threatened or Endangered. Federal Register 72:32589.
Wagner, E.J. and R.W. Oplinger. 2013. Wild fish traps in Utah: A review of their history, management, and fish production. Publication Number: 13-12. Utah Division of Wildlife Resources. Salt Lake City, Utah.
Young, M.K. 1995. Colorado River cutthroat trout. Conservation assessment for inland cutthroat trout. United States Forest Service, Fort Collins, CO. 16-23.
Young, M.K. 1996. Summer movements and habitat use by Colorado River cutthroat trout (Oncorhynchus clarki pleuriticus) in small, montane streams. Canadian Journal of Fisheries and Aquatic Sciences 53: 1403-1408. https://doi.org/10.1139/f96-054
Young, M.K., R.B. Rader and T.A. Relish. 1997. Influence of macroinvertebrate drift and light on the activity and movement of Colorado River cutthroat trout. Transactions of the American Fisheries Society 126:428-437. https://doi.org/10.1577/1548-8659(1997)126<0428:IOMDAL>2.3.CO;2
Young, M.K. 1998. Absence of autumnal changes in habitat use and location of adult Colorado River cutthroat trout in a small stream. Transactions of the American Fisheries Society 127:147-151. https://doi.org/10.1577/1548-8659(1998)127<0147:AOACIH>2.0.CO;2
Young, M.K. 2011. Generation-scale movement patterns of cutthroat trout (Oncorhynchus clarkii pleuriticus) in a stream network. Canadian Journal of Aquatic and Fisheries Sciences 68: 941-951. https://doi.org/10.1139/f2011-023
Contact
Feel free to contact me if you have any questions or comments
Green River Cutthroat Trout Links
My Green River Cutthroat Trips
Utah Cutt Slam - Colorado River Cutthroat Trout
Colorado Department of Parks and Wildlife - Colorado River Cutthroat Trout
Uinta-Wasatch-Cache National Forest
Medicine Bow-Routt National Forests
Western Native Trout Initiative - Colorado River Cutthroat Trout
Trout Unlimited - Colorado River Cutthroat Trout
Native Trout Links
California Heritage Trout Challenge
Truchas Mexicanas' - Native Trout of Mexico
Balkan Trout Restoration Group
Trout and Seasons of the Mountain Village - About Japanese Trout
Fly Fishing Blogs
Dave B's Blog: Fly Fishing for Native Trout
The Search for Native Salmonids
Conservation Links
Western Native Trout Initiative
Fly Fishing Links
Fishing Art Links
Americanfishes.com - Joseph R. Tomelleri